Microtechniques: Microtechniques are specialized methods used to prepare biological samples for microscopic examination.
These techniques involve a series of steps that preserve the structure of the sample, allow for thin sectioning, and stain specific components for better visualization under a microscope.
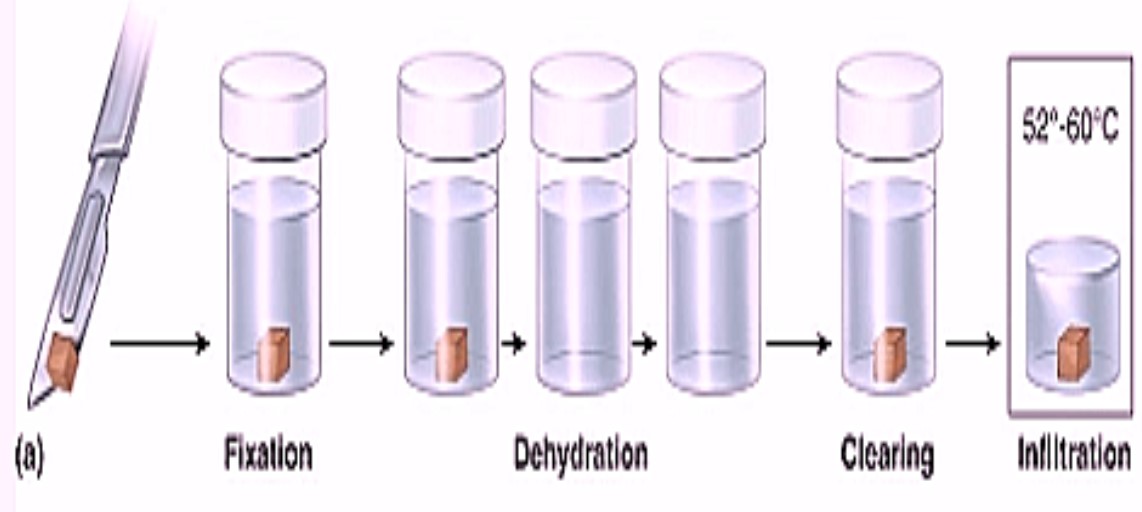
Rat tissues are fixed in Bouin’s Fluid. Fixation is essential as the tissue becomes hard and prevents changes which take place in tissue after death of animal. It fixes the tissues as they were in the live condition. Generally Bouin’s fluid is used for fixing tissues.
Preparation of Bouin’s fluid: Dissolve 1gm of picric acid in 250 cc of 90% alcohol. Then add 0.5ml acetic acid to preserve the fixative. Fixation is done for minimum 24 hours after which tissues can be processed. ‘Microtechniques’
Dehydration:
Ddehydration means removal of water from the tissues. Dehydration is done by alcohol, make small pieces of the tissue and pass in various grades of alcohol 30%, 50%, 70%, 90% and 100%.
Decaying of tissue takes place due to water, hence its removal is necessary. In dehydration water is replaced by alcohol in the tissues which prevents decaying of the cell and maintains the structure and shape of the cells as they were in live condition.
After dehydration keep the tissues for 12 hours in Lithium carbonate solution which removes excess of fixative (Bouin’s Fluid). ‘Microtechniques’
After lithium carbonate tissues are kept in xylene for 10 minutes for clearing.
Block Preparation:
It includes cold impregnation and hot impregnation
Cold Impregnation: cut wax into very small and thin pieces. Add xylene to it and keep it for one hour. After, stir the solution. Wax is completely dissolved in xylene and a paste of wax is formed. Then transfer the tissues from the xylene to this paste of wax. Keep in it for 12 hours. Alcohol in the tissue is then replaced by wax.
Hot impregnation: take wax in a beaker and keep it in oven till it melts. Set the oven at 580C. After melting filter the wax with filter paper in another conical flask. Transfer the tissues in this filtered wax and keep it for 1 hour. This will make the tissue hard and ready to cut on the microtome.
Blocks are prepared in ‘L’ blocks which are of metal. Two L blocks are place side by side so that a square forms. Apply glycerin to the blocks. Pour the filtered wax in the block and after 30 seconds transfer the tissues in the blocks. Let it cool for 1 hour. After one hour remove the metal L block and you wax block is ready with tissue inside.
Sectioning/Microtomy:
Trim the block and make it a perfect square. Fix the block to the block holder and attach it to microtome. Sections are cut on rotary microtome. Thin sections of 7microns are cut. A long ribbon of sections is formed. ‘Microtechniques’
Take clean slide, apply and rub some egg albumen to it and then place the wax tissue ribbon on it. Add some water drops between the slide and the ribbon and heat it gently for 2 second atleast two times.
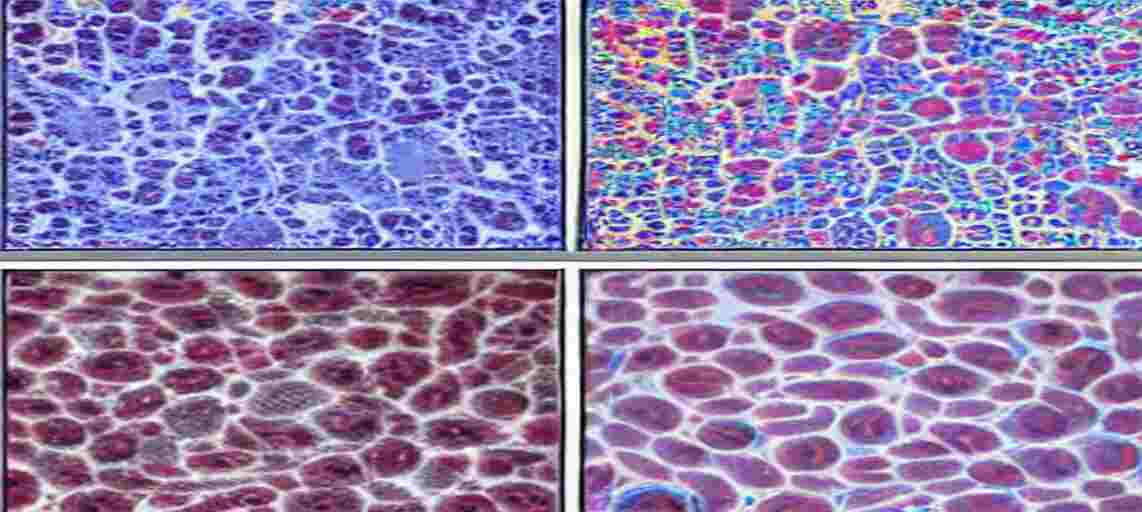
Staining
Staining means colouring the tissue cells and their organelles. Generally Hematoxylin and Eosin stains are used.
Preparation of Stains Hematoxylin:
- Hematoxylin – 4gm
- Absolute alcohol – 25 cc
- Alum – saturated
- Glycerine – 100 cc
- Methyl alcohol – 100cc
Mix 4 gm of hematoxylin with 25 cc of absolute alcohol and then add ammonium alum to it and keep it for four days and then add glycerine and methyl alcohol to it. Filter and use it. ‘Microtechniques’
Eosin: Dissolve 0.5 gm of eosin in 100 cc of alcohol.
Staining: Stain the slide by passing it into following grades
Xylene – 3 minutes
100% alcohol- 3 minutes
90% alcohol- 5 minutes
70% alcohol- 5 minutes
50% alcohol- 5 minutes
30% alcohol- 5 minutes
Stain in Hematoxylin – 15 min
Wash in water – one wash/one dip
30% alcohol- 5 minutes
50% alcohol- 5 minutes
70% alcohol- 5 minutes
Eosin – 15 minutes
90% alcohol- 5 minutes
100% alcohol- 5 minutes
Xylene
Mount the tissue with DPX and observe under microscope.