Carbohydrates: The carbohydrates are a group of naturally occurring carbonyl compounds (aldehydes or ketones) that also contain several hydroxyl groups. Carbohydrate are the most abundant organic molecules in nature and also referred to as “saccharides”. The carbohydrates which are soluble in water and sweet in taste are called as “sugars”.
Structure of Carbohydrates
Carbohydrates consist of carbon, hydrogen, and oxygen. The general empirical structure for carbohydrates is (CH2O)n. They are organic compounds organized in the form of aldehydes or ketones with multiple hydroxyl groups coming off the carbon chain. The building blocks of all carbohydrates are simple sugars called monosaccharides. A monosaccharide can be a polyhydroxy aldehyde (aldose) or a polyhydroxy ketone (ketose)
The carbohydrates can be structurally represented in any of the three forms:
- Open chain structure
- Hemi-acetalstructure
- Haworth structure
Open chain structure: It is the long straight-chain form of carbohydrates.
Hemi-acetal structure: Here the 1st carbon of the glucose condenses with the -OH group of the 5th carbon to form a ring structure.
Haworth structure: It is the presence of the pyra nose ring structure
Classification of Carbohydrates
Carbohydrates are classified into four major groups based on the degree of polymerization these are monosaccharides, disaccharides, oligosaccharides, and polysaccharides which is shown in diagram with examples.
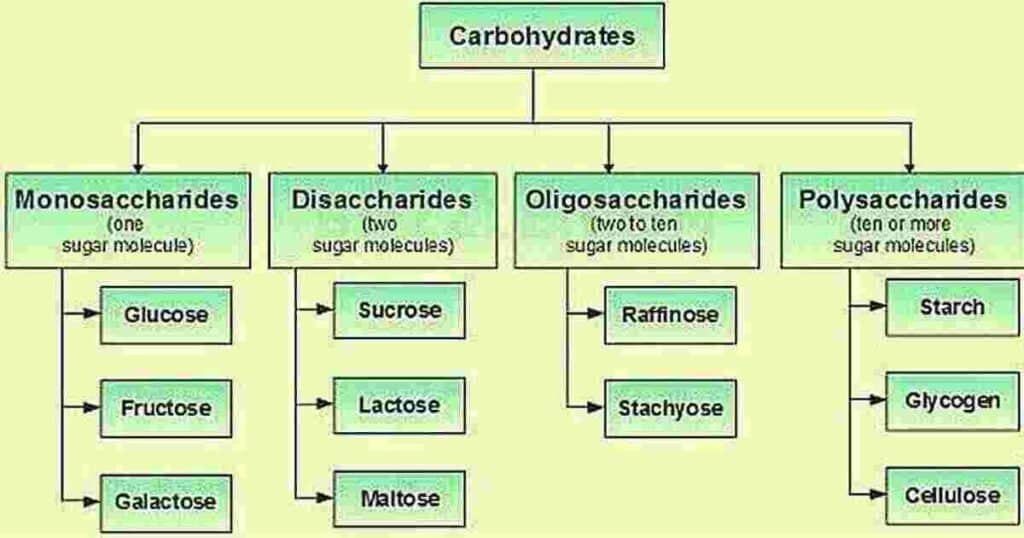
Monosaccharides
The simple carbohydrates include single sugars (mono saccharides) and polymers, oligosaccharides, and polysaccharides. Simplest group of carbohydrates and often called simple sugars since they cannot be further hydrolyzed. Colorless, crystalline solid which are soluble in water and insoluble in a non-polar solvent. These are compound which possesses a free aldehyde or ketone group. The general formula is Cn(H2O)nor CnH2nOn. They are classified according to the number of carbon atoms they contain and also on the basis of the functional group present. The monosaccharides thus with 3,4,5,6,7… carbons are called trioses, tetroses, pentoses, hexoses, heptoses, etc., and also as aldoses or ketoses depending upon whether they contain aldehyde or ketone group.
Examples: Glucose, Fructose, Erythrulose, Ribulose.
Chemical Structure of Some Monosaccharides
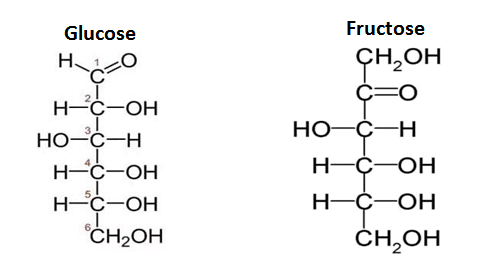
Properties of Monosaccharides
- Most monosaccharides have a sweet taste (fructose is sweetest;73% sweeter than sucrose).
- They are solids at room temperature.
- They are extremely soluble in water
- Glucose can dissolve in minute amounts of water to make a syrup(1 g / 1 ml H2O).
Disaccharides
Two monosaccharides connected by a glycosidicbond. Disaccharide, also called double sugar, any substance that is composed of two molecules of simple sugars (monosaccharides) linked to each other. Disaccharides are crystalline water-soluble compounds. The monosaccharides within them are linked by a glycosidicbond (or glycosidiclinkage), the position of which may be designated α-or β-or a combination of the two (α-,β-). Glycosidicbonds are cleaved by enzymes known as glycosidases. The three major disaccharides are sucrose, lactose, and maltose. Sucrose, which is formed following photosynthesis in green plants, consists of one molecule of glucose and one of fructose bonded via an α-,β-linkage. Lactose (milk sugar), found in the milk of all mammals, consists of glucaose and galactose connected by a β-linkage. Maltose, a product of the breakdown of starches during digestion, consists of two molecules of glucose connected via an α-linkage. Another important disaccharide, trehalose, which is found in single-celled organisms and in many insects, also consists of two molecules of glucose and an α-linkage, but the linkage is distinct from the one found in maltose.
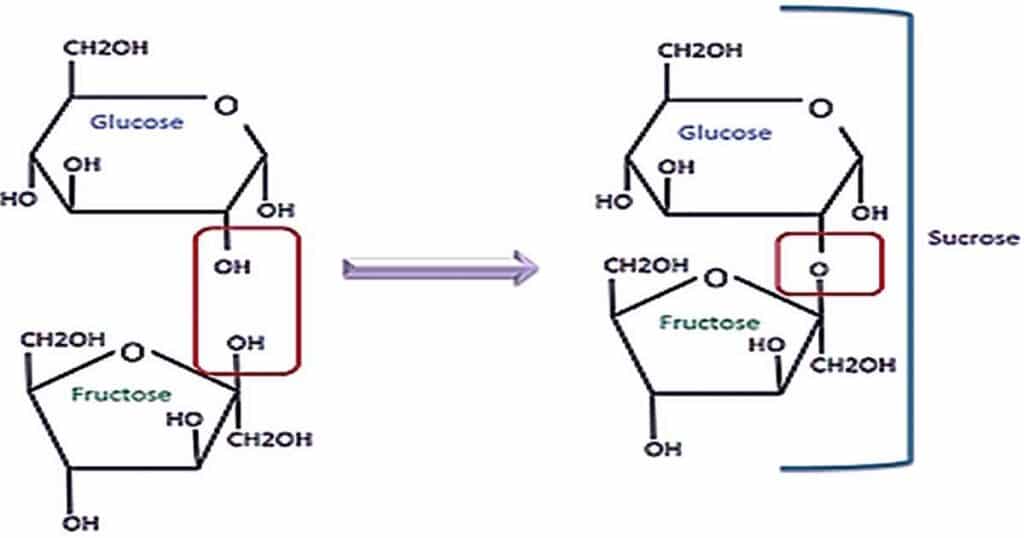
Significance of Disaccharides
More specifically, a disaccharide results when two monosaccharides are joined in a chemical process called dehydration synthesis, which causes two monosaccharides to combine, losing a water molecule in the process. This process is also known as a condensation reaction. Here we see an example of the formation of the disaccharide sucrose, formed from the combination of the monosaccharides fructose and glucose.
Oligosaccharides
Oligosaccharides are compound sugars that yield 2 to 10 moleculesof the same or different monosaccharides on hydrolysis. The monosaccharide units are joined by glycosidiclinkage. Based on the number of monosaccharide units, it is furtherclassified as disaccharide, trisaccharide, tetrasaccharideetc. Oligosaccharides yielding 2 molecules of monosaccharides onhydrolysis is known as a disaccharide, and the ones yielding 3 or 4
Structure of Oligosaccharides
They are normally present as glycans: oligosaccharide chains linked to lipids or to compatible amino acid side chains in proteins, by N-or O-glycosidicbonds. N-Linked oligosaccharides are always pentasaccharidesattached to asparagine via a beta linkage to the amine nitrogen of the side chain. Alternately, O-linked oligosaccharides are generally attached to threonine or serine on the alcohol group of the side chain. Not all natural oligosaccharides occur as components of glycoproteins or glycolipids. Some, such as the raffinoseseries, occur as storage or transport carbohydrates in plants. Others, such as maltodextrinsor cellodextrins, result from the microbial breakdown of larger polysaccharides such as starch or cellulose.
N-Linked oligosaccharides: N-Linked glycosylation involves oligosaccharide attachment to asparagine via a beta linkage to the amine nitrogen of the side chain. The process of N-linked glycosylation occurs cotranslationally, or concurrently while the proteins is being translated. Since it is added cotranslationally, it is believed that N-linked glycosylation helps determine the folding of polypeptides due to the hydrophilic nature of sugars. All N-linked oligosaccharides are pentasaccharides: five monosaccharides long. An example of an N-linked oligosaccharide
O-linked Oligosaccharides: It participate in glycosylation are attached to threonine or serine on the hydroxyl group of the side chain. O-linked glycosylation occurs in the Golgi apparatus, where monosaccharide units are added to a complete polypeptide chain. Cell surface proteins and extracellular proteins are O-glycosylated.
Polysaccharides
They are also called as “glycans”. Polysaccharides contain more than 10 monosaccharide units andcan be hundreds of sugar units in length. They yield more than 10 molecules of monosaccharides on hydrolysis. Polysaccharides differ from each other in the identity of their recurring monosaccharide units, in the length of their chains, in the types of bond linking units and in the degree of branching. They are primarily concerned with two important functions ie. Structural functions and the storage of energy.
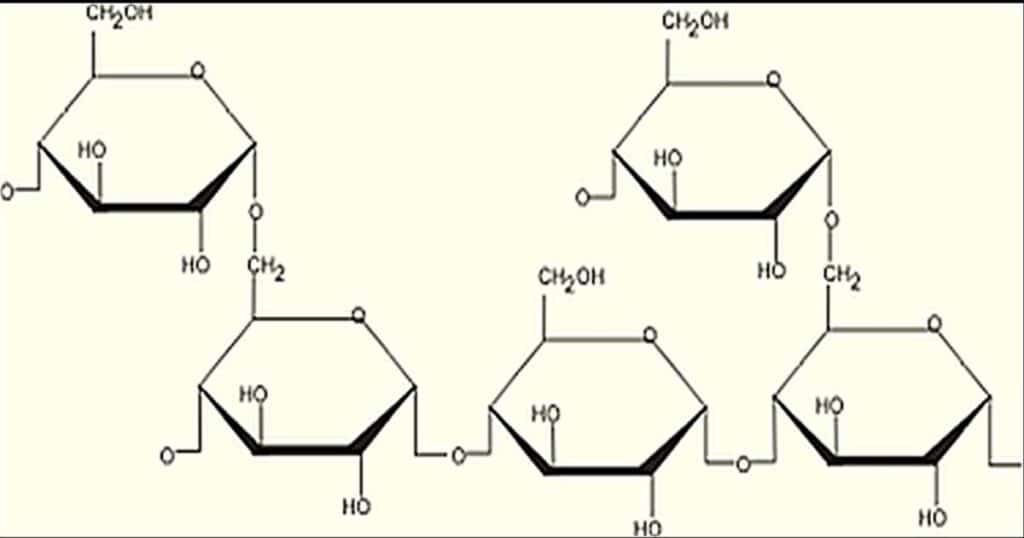
Function of Polysaccharides
1. Polysaccharides such as starch, glycogen and dextransare all stored in the liver and muscles to be converted to energy for later use.
2. Amylose and Amylopectin are polysaccharides of starch. Amylose has a linear chain structure made up of hundreds of glucose molecules that is linked by a alpha 1,4 glycosidiclinkage
3. Glycogen is found in animals, and it is branched like amylopectin. It is formed by mostly alpha 1,4 glycosidiclinkages but branching occurs more frequently than in amylopectin as alpha 1,6 glycosidiclinkages occur about every ten units.
4. Other polysaccharides have structural functions. For example, cellulose is a major component in the structure of plants. Cellulose is made of repeating beta 1,4-glycosidic bonds.
Function of Carbohydrate
1. Carbohydrates are widely distributed molecules in plant and animal tissues. In plants and arthropods, carbohydrates from the skeletal structures, they also serve as food reserves in plants and animals. They are important energy source required for various metabolic activities.
2. Living organisms use carbohydrates as accessible energy to fuel cellular reactions. They are the most abundant dietary source of energy (4kcal/gram) for all living beings.
3. Carbohydrates along with being the chief energy source, in many animals, are instant sources of energy. Glucose is broken down by glycolysis/ Kreb’scycle to yield ATP.
4. Serve as energy stores, fuels, and metabolic intermediates. It is stored as glycogen in animals and starch in plants.
5. Stored carbohydrates act as an energy source instead of proteins.
6. They form structural and protective components, like in the cell wall of plants and microorganisms. Structural elements in the cell walls of bacteria (peptidoglycan or murein), plants (cellulose) and animals (chitin).
7. Carbohydrates aid in the regulation of nerve tissue and is the energy source for the brain.
8. Carbohydrates get associated with lipids and proteins to form surface antigens, receptor molecules, vitamins, and antibiotics.
9. Formation of the structural framework of RNA and DNA (ribonucleic acid and deoxyribonucleic acid).
10. They are linked to many proteins and lipids. Such linked carbohydrates are important in cell-cell communication and in interactions between cells and other elements in the cellular environment.
11. In animals, they are an important constituent of connective tissues.
12. Also, they help in the modulation of the immune system.
13. Examples of Homo polysaccharidesare starch, glycogen, cellulose, pectin.
14. Heteropolysaccharidesare Hyaluronic acid, Chondroitin monosaccharides are known as trisaccharides and tetrasaccharides respectively and so on.
15. The general formula of disaccharides is Cn(H2O)n-1 and that of trisaccharidesis Cn(H2O)n-2and so on.
16. Examples: Disaccharides include sucrose, lactose, maltose,etc. Trisaccharides are Raffinose, Rabinose.
Physical Properties of Carbohydrate
•Stereoisomerism –Compound shaving the same structural formula but they differ in spatial configuration. Example: Glucose has two isomers with respect to the penultimate carbon atom. They are D-glucose and L-glucose.
•Optical Activity –It is the rotation of plane-polarized light forming(+) glucose and (-) glucose.
•Diastereoisomers –It the configurational changes with regard to C2, C3, or C4 in glucose. Example: Mannose, galactose.
•Annomerism–It is the spatial configuration with respect to the first carbon atom in aldoses and second carbon atom in ketoses.