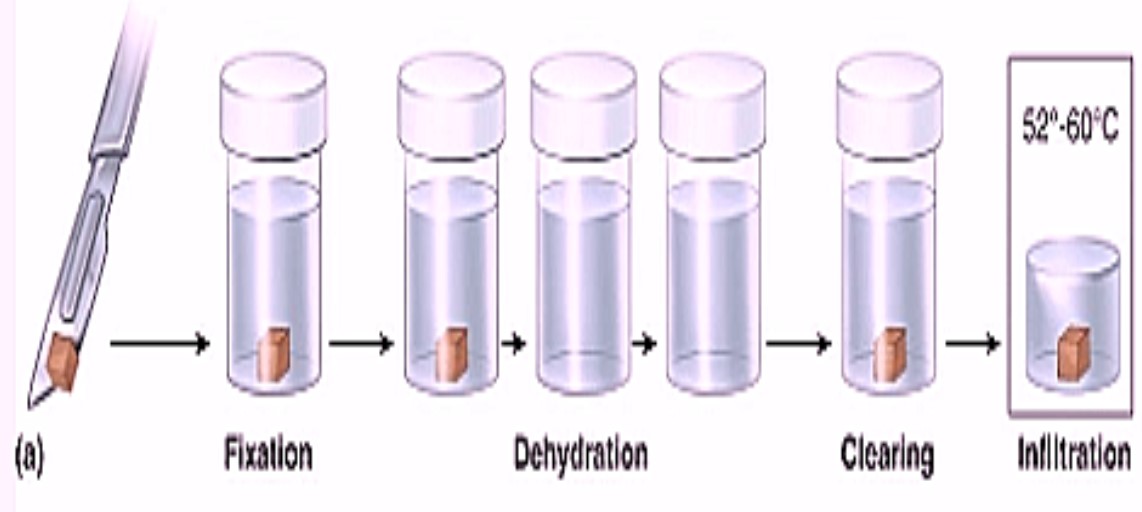
Fixation: In the fields of histology, pathology, and cell biology, fixation is a critical step in the preparation of histological sections by which biological tissues are preserved from decay, thereby preventing autolysis or putrefaction.
Fixation Definition:
It is a complex series of chemical events which brings about changes in the various chemical constituents of cell like hardening, however the cell morphology and structural detail is preserved.
Principles of Fixation
Fixation results in denaturation and coagulation of protein in the tissues. The fixatives have a property of forming cross links between proteins, thereby forming a gel, keeping everything in their in vivo relation to each other
PROPERTIES OF FIXATIVES AND FACTORS AFFECTING FIXATION
1. Coagulation and precipitation of proteins in tissues.
2. Penetration rate differs with different fixatives depending on the molecular weight of the fixative
3. pH of fixatives – Satisfactory fixation occurs between pH 6 and 8. Outside this range, alteration in structure of cell may take place.
4. Temperature – Room temperature is alright for fixation. At high temperature there may be distortion of tissues.
5. Volume changes – Cell volume changes because of the membrane permeability and inhibition of respiration.
6. An ideal fixative should be cheap, nontoxic and non-inflammable. The tissues may be kept in the fixative for a long time.
Types of Fixation
Immersion fixation
Perfusion fixation
Vapour fixation
Coating/Spray fixation
Freeze drying
Microwave fixation/Stabilization The most commonly used technique is simple immersion of tissues/smears in an excess of fixative. For all practical purposes immersion fixatives are most useful. These may be divided into routine and special.
Simple Fixatives
1. Formaldehyde: Commercially available solution contains 35%-40% gas by weight, called as formalin. Formaldehyde is commonly used as 4% solution, giving 10% formalin for tissue fixation. Formalin is most commonly used fixative. It is cheap, penetrates rapidly and does not over- harden the tissues. The primary action of formalin is to form additive compounds with proteins without precipitation. Formalin brings about fixation by converting the free amine groups to methylene derivatives.
If formalin is kept standing for a long time, a large amount of formic acid is formed due to oxidation of formaldehyde and this tends to form artefact which is seen as brown pigment in the tissues. To avoid this buffered formalin is used.
2. Absolute alcohol – it may be used as a fixative as it coagulates protein. Due to its dehydrating property it removes water too fast from the tissues and produces shrinkage of cells and distortion of morphology. It penetrates slowly and over-hardens the tissues.
3. Acetone – Sometimes it is used for the study of enzymes especially phosphatases and lipases. Disadvantages are the same as of alcohol.
4. Mercuric chloride – It is a protein precipitant. However it causes great shrinkage of tissues hence seldom used alone. It gives brown colour to the tissues which needs to be removed by treatment with Iodine during dehydration.
5. Potassium dichromate – It has a binding effect on protein similar to that of formalin. Following fixation with Potassium dichromate tissue must be well washed in running water before dehydration.
6. Osmic acid – It is used for fixation of fatty tissues and nerves.
7. Chromic acid – It precipitates all proteins and preserves carbohydrates. Tissues fixed in chromic acid also require thorough washing with water before dehydration.
8. Osmium tetraoxide – It gives excellent preservation of cellular details, hence used for electron-microscopy.
9. Picric acid – It precipitates proteins and combines with them to form picrates. Owing to its explosive nature when dry; it must be kept under a layer of water. Tissue fixed in picric acid also require thorough washing with water to remove colour. Tissue can not be kept in picric acid more than 24 hrs.
Compound Fixatives
1. Formal saline – It is most widely used fixative. Tissue can be left in this for long period without excessive hardening or damage. Tissues fixed for a long time occasionally contain a pigment (formalin pigment). This may be removed in sections before staining by treatment with picric alcohol or 10% alcoholic solution of sodium hydroxide. The formation of this pigment can be prevented by neutralizing or buffering the formal saline. Fixation time – 24 hours at room temprature
2. Formal calcium – Useful for demonstration of phospholipids. Fixation time-24 hours at room temperature
3. Zenker’s fluid – It contains mercuric chloride, potassium-di-chromate, sodium sulphate and glacial acetic acid. Advantages – even penetration, rapid fixation
Disadvantages – After fixation the tissue must be washed in running water to remove excess dichromate. Mercury pigment must be removed with Lugol’s iodine.
4. Zenker’s formal (Helly’s fluid) – In stock Zenker’s fluid, formalin is added instead of acetic acid. Advantages – excellent microanatomical fixative especially for bone marrow, spleen & kidney.
5. Bouins fluid – It contains picric acid, glacial acetic acid and 40% formaldehyde.
Advantages – (a) Rapid and even penetration without any shrinkage. (b) Brilliant staining by trichrome method. It is routinely used for preservation of testicular biopsies.
Points to Remember
1. 10% buffered formalin is the commonest fixative.
2. Tissues may be kept in 10% buffered formalin for long duration.
3. Volume of the fixative should be atleast ten times of the volume of the specimen. The specimen should be completely submerged.
4. Special fixatives are used for preserving particular tissues.
5. Formalin vapours cause throat/ eye irritation hence mask/ eye glasses and gloves should be used.
6. Tissues should be well fixed before dehydration.
7. Penetration of fixatives takes some time. It is necessary that the bigger specimen should be given cuts so that the central part does not remain unfixed.
8. Mercury pigment must be removed with Lugol’s iodine.
9. Biopsies cannot be kept for more than 24 hours in bouin’s fluid without changing the alcohol.
10. Glutaraldehyde and osmion tetraoxide are used as fixatives for electron microscopy.
Most Commonly used Fixatives in the Laboratory are
10% Formalin
Formaldehyde (40%) – 10 ml
Distilled water – 90 ml
Formal Saline
Formaldehyde (40%) – 100 ml
Sodium Chloride – 9 gm
Distilled Water – 900 ml
10% Buffered Formalin
Formaldehyde (40%) – 10 ml
Sodium dihydrogen phosphate – 0.4 gm
Disodium hydrogen phosphate (anhydrous) – 0.65 gm
Distilled water – 90 ml
The advantage of this fixative is that it prevents the formation of formalin pigment
Bouin’s solution
Saturated picric acid (1.2 gm/ 100 ml) – 750 ml
Formaldehyde (40%) – 250 ml
Glacial acetic acid – 50 ml
Alcoholic formaldehyde
40% formaldehyde – 100 ml
95% alcohol – 900 ml
0.5 g calcium acetate may be added to this mixture to ensure neutrality
Alcohol containing fixatives
Carnoy’s fixatives –
Absolute ethanol – 60 ml
Chloroform – 30 ml
Glacial acetic acid – 10 ml
Mercury salt containing fixatives
Zenker’s fluid
Distilled water – 950 ml
Potassium dichromate – 25 gm
Mercuric Chloride – 50 gm
Glacial acetic acid – 50 gm
B5 fixative
Stock reagent A
Mercuric chloride – 60 g
Sodium acetate – 12.5 g
Distilled water – 1000 ml
Stock Reagent B
10% buffered neutral formalin
Working Solution
Stock reagent A – 90 ml
Stock reagent B – 10 ml
Fixation time – 5-8 hrs
Adequate time should be given for fixation. Formalin fixation should ideally be given for at least 8 hours before processing. (Not the whole specimen but the cut sections).