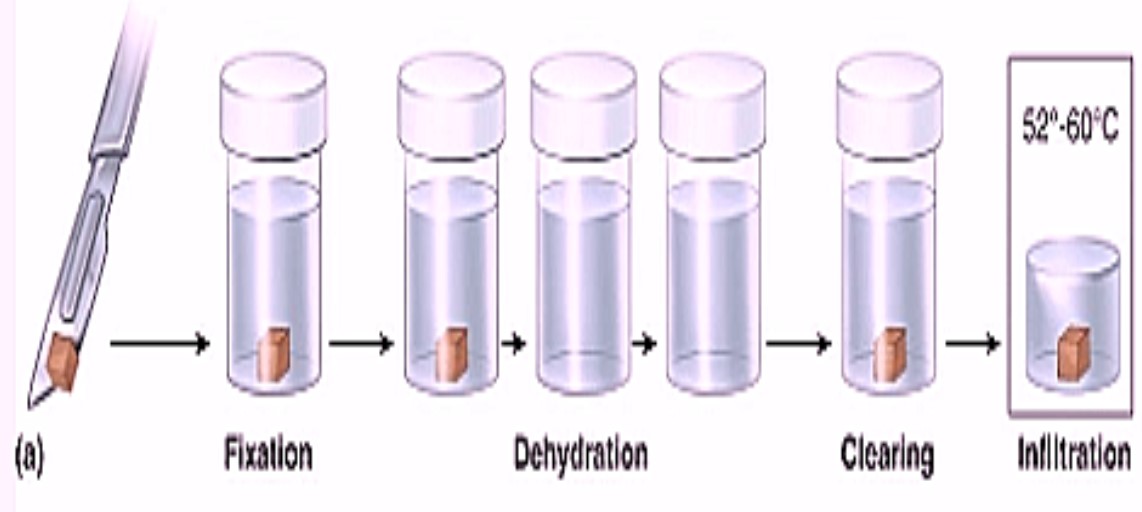
Tissue Processing for histopathology: Tissue processing is a critical step in histopathology, transforming biological tissue into a form suitable for microscopic examination. This involves a series of steps that preserve tissue structure and prepare it for sectioning and staining.
What is tissue processing?
The technique of getting fixed tissues into paraffin is called tissue processing. It describes the steps required to take animal tissues from fixation to the state where it is completely infiltrated with a suitable wax i.e. paraffin wax and can be embedded and ready for section cutting on microtome.
Significance of Tissue Processing
Preservation of tissue morphology: Proper tissue processing maintains the original structure of cells and tissues. 1. Histology, Staining – StatPearls – NCBI Bookshelf www.ncbi.nlm.nih.gov
Enabling microscopic examination: The process creates tissue sections thin enough for light to pass through, allowing detailed study.
Accurate diagnosis: High-quality tissue preparation is essential for pathologists to make accurate diagnoses. 1. An Introduction to Specimen Processing – Leica Biosystems www.leicabiosystems.com
Research applications: Processed tissues are used in various research areas, including histology, immunohistochemistry, and molecular pathology.
Tissue Processing Techniques for Histopathology
Tissue processing techniques consisting the fixation, dehydration, clearing, infiltration, embedding, sectioning and mounting.
1. Fixation: This is the initial step where tissue is preserved to prevent decomposition. Fixation is the preservation of biological tissues from decay due to autolysis or putrefaction. It terminates any ongoing biochemical reactions and may also increase the treated tissues mechanical strength or stability. Tissue fixation is a critical step in the preparation of histological sections, its broad objective being to preserve cells and tissue components and to do this in such a way as to allow for the preparation of thin, stained sections.
Common fixatives include formalin (formaldehyde solution), alcohol, and glutaraldehyde.
Fixation cross-links proteins, preventing autolysis and putrefaction. 1. Intro to Tissue Fixation in Histology: Types, Methods & More – Leica Biosystems www.leicabiosystems.com
2. Dehydration: It is the process of removing water from tissues. It is important because paraffin is not miscible with water. Dehydration is usually complete when less then 3-4% of water remains in the tissues. Time required for this depends on: 1. Permeability of tissues 2. Continuous rotation of fluid to prevent stagnation of fluid around tissues 3. Temperature 4. Vacuum applied ‘Tissue Processing for histopathology’
Dehydrants: Ethyl alcohol, Methyl alcohol, Butyl alcohol and Isopropyl alcohol.
The most commonly used dehydrant is ethyl alcohol.
Alcohol Method: The tissues are passed through a series of progressively more concentrated alcohol baths. Concentration of first alcohol bath depends on the fixative and size and type of the tissue, e.g. delicate tissue needs lower concentration of alcohol and smaller interval between two strengths of alcohol. Water is removed from the tissue using a graded series of alcohol solutions (70%, 95%, and 100%). Usually tissues are kept in each solution for 40 to 60 minutes.
Usually 70% alcohol is employed as the first solution and100% as the last solution. After about 40 tissues have passed through the first change of alcohol, it is discarded and all the other changes are brought one step lower. Absolute alcohol at the end is always fresh.
Use of copper sulphate in final alcohol: A layer of anhydrous CuSO4 is placed at the bottom of a dehydrating bottle or beaker and is covered with 2-3 filter paper of approximate size to prevent staining of the tissue. ‘Tissue Processing for histopathology’
Anhydrous CuSO4 removes water from alcohol as it in turn removes it from tissues.
Anhydrous CuSO4 is white in colour while the hydrated form is blue. Therefore, it acts as an indicator for the presence of water.
Advantage of CuSO4 –
1. Rapid dehydration
2. Prolongs life of alcohol
3. Blue colouration of CuSO4 indicates that both alcohol and CuSO4 should be changed.
Acetone – Acetone is clear colorless inflammable fluid which is miscible with water, ethanol. It is used for complete dehydration. Four changes of acetone of half an hour or two changes of one hour are given to achieve complete dehydration of tissues.
Advantages: Rapid action, Easily removed by most clearing agents, Less expensive Disadvantages: Highly volatile, Causes shrinkage and brittleness of tissues
3. Clearing: Dissolves lipid more than ethanol Clearing – Clearing is a process which leaves the tissues clear and transparent. This term relates to the appearance of the tissues after the dehydrating agent has been removed. If the refractive index of the clearing agent is similar to the protein of tissue the tissue becomes transparent. The end point of clearing can be noted by the transparent appearance of the tissue.
Thus clearing serves two purposes
1. Removes alcohol to make paraffin impregnation complete
2. Acts as solvent for the mounting media which renders the tissues transparent and improves the refractive index, making microscopic examination easier.
Clearing Agents
Xylene – It is colourless and most commonly used. Two changes of one hour each are given to get the end point. Prolonged treatment hardens the tissues. It is not preferred for brain tissue.
Other Clearing Agents: Toluene, Dioxane, Cedarwood oil, Cloroform, Benzene
Carbol-xylene – clears rapidly, it is kept reserved for material difficult to clear.
4. Infiltration and Impregnations: After clearing, tissues are transferred to molten paraffin wax for filtration and impregnation. During this process clearing agent diffuses out and molten wax is infiltrated. The wax which has infiltrated in the tissue gets deposited. This process is called impregnation. Routinely two changes are given in the wax to get proper impregnation. The duration and number of changes required for thorough impregnation of tissue depends on –
1. Size and type of tissues-Longer time is required for thicker tissues. Vacuum reduces the time required for complete impregnation.
2. Clearing agent employed
3. Use of vacuum imbedding Tissue processing may be performed manually or with the help of automated tissue processor. Routinely 12 containers containing different solutions are used for processing in the following order
10% formalin – container no 1, 2
50% alcohol – container no 3
90% alcohol – container no 4 & 5
Absolute Alcohol – container no 6
Acetone – container no 7 & 8
Xylene – container no 9 & 10
Paraffin Wax – container no 11 & 12
5. Embedding: The paraffin wax-infiltrated tissue is placed in a mold and allowed to solidify. 1. An Introduction to Specimen Processing – Leica Biosystems www.leicabiosystems.com
This forms a tissue block ready for sectioning. 1. Tissue Processing, Embedding and Sectioning – Oxford Cancer www.cancer.ox.ac.uk
6. Sectioning: The tissue block is trimmed and cut into thin sections (usually 4-6 micrometers) using a microtome. 1. Introductory Chapter: Histological Microtechniques – IntechOpen www.intechopen.com
These sections are then floated on a water bath to flatten. 1. Instrumentation for Microtomy: Flotation Bath – LabCE.com, Laboratory Continuing Education www.labce.com
7. Mounting: The sections are placed on glass slides and dried. 1. Proper Slide Drying for Paraffin-Sectioned Slides – LabCE.com, Laboratory Continuing Education www.labce.com